Water from lakes and streams can contain a lot of things that can make you sick. Making drinking water safe when camping, on the trail, or simply traveling, has become big business with many options. A safe solution is easier and cheaper than you might think. Minnesota Sea Grant’s April 2023 Director’s Column has information to help keep you safe and hydrated.
Image credit: Adobe Stock/kaininstudio ©
The changing world of natural water
In 1904, my family lived on a lake in Itasca County and hauled drinking water in buckets from the lake. In the 1960s and 1970s, when my dad put up a cabin on his tiny teaching salary, he ran a flexible black pipe from twenty feet offshore to his cabin, put a foot valve on it, connected an old Rapidayton jet pump, and we had water! We drank it, we washed with it, we bathed in it (when we didn’t bathe in the lake), and we flushed with it (as soon as we could afford a septic tank). None of our family ever got sick from drinking that water.
Thirty years after that, when neighbors around the lake became concerned about decreases in lake water clarity, my dad, who was a biology professor, tested the water and found fecal coliform bacteria; the kind that indicated that people might get sick from drinking or swimming in the water. After my dad saw those test results, he had a deep well installed. Deep groundwater is purer than surface water because it trickles down through clean gravel for decades.
In 2022, when I tested the water in the same lake, I rarely found a sample without those bacteria and sometimes, when there were a lot of people around, there were a lot of those bacteria. There are few surveys of waterborne pathogens in Minnesota’s lakes and streams, but outbreaks of illness from contact with recreational waters are common in Minnesota (1) and illness-causing organisms are frequently found even in deep Minnesota public water supply wells (2).
Our Minnesota lake water still may look clear, but it may no longer be safe to drink straight from the lake. A 2019 Minnesota Water Values Resident Survey Report by Mae Davenport, professor and director of the University of Minnesota Center for Changing Landscapes shows that the water values most important to Minnesotans were clean and safe drinking water. The same concern is likely true whether you are in southern or northern Minnesota or up in the boundary waters. It is also true if you live in or visit a place that has had a natural or human-made disaster. Diseases like salmonellosis (Salmonella infection), giardiasis (Giardia infection) , or avian flu are becoming uncomfortably common and we all need to be careful to avoid spoiling our few days in the wild with an avoidable and sometimes very serious illness.
Water safety problems are getting much worse as the climate changes to warmer and stormier conditions (3). When people go canoeing, camping, or backpacking, one of the three most important pieces of gear they should pack is some means of purifying drinking water. The same is true for people who travel internationally, especially if they are going to a place where water or weather disasters are common or drinking water treatment questionable.
Water is our body’s most important nutrient
Water makes up about 60% of your body weight and nearly all of your body’s major systems depend on water to function and survive according to the Mayo Clinic. Water is our body’s most important nutrient (4). Supplying enough water when you are canoeing, camping, or traveling is important to your health.
Effects of poor hydration:
- Impedes your ability to regulate body temperature
- Worsens your physical performance
- Degrades your abilities to think and reason
- Interferes with your body’s ability to digest food and extract energy from that food
- Disrupts your body’s ability to get rid of toxins
- Can increase heart rate and blood pressure
Poor hydration is associated with:
- Headaches including migraines
- A reduction in our skin’s ability to retain water
- Serious diseases including urinary tract infections, high blood pressure, coronary heart disease, deep vein thrombosis, stroke (5).
It is important to stay hydrated, especially when engaging in strenuous activities outside of a person’s normal routine (6).
How much water do you need?
Scientists reported in a 2005 scientific paper in the journal Nutrition Reviews that:
- Adult men use about 4 U.S. quarts (3.7 liters; 125 oz.) per day.
- Adult women use about 3 U.S. quarts (2.7 liters; 90 oz.) per day (7).
Because about 22% of a person’s water needs are met by water from food (5), that means men and women need to drink 3 quarts and 2.5 quarts per day, respectively, if they are moderately active. Of course, increases in heat and activity can increase water intake needs substantially. In weather warm enough to lead to perspiration, very active adults engaging in intense activity need more than double that amount of water or about 5-6 quarts of water per day.
The amount of water you will need to purify for a week-long wilderness trip is 18-42 quarts per person (4.5-10.5 gallons each) for the week. If we think of a three-adult canoe trip in the boundary waters that is 14-40 gallons of purified water. That would weigh 112-334 lbs. It is far simpler to purify water found in nature than to pack it in.
Why purify natural water?
This Minnesota Sea Grant Director’s Column explains how to remove disease organisms from water. If the water has poisonous chemicals in it, much more serious treatment is needed. High concentrations of harmful chemicals are rare in wild places not receiving industrial or municipal waste and so are probably not an immediate concern. Check the Minnesota impaired waters list to see if where you plan to go has water that fails to meet one or more water quality standards and is considered impaired. If your destination has chemically impaired waters, you will need expensive and sophisticated equipment or it may be best to carry in pure or purified water.
Most things in natural water that can make you sick are living things. From the smallest to the largest, the things that make us sick are viruses, bacteria, protozoans, and worms.
Viruses
People often do not think about catching viruses from drinking water but there are many of them and their numbers are increasing in natural waters. Viruses can cause humans inconvenience, discomfort and/or long-term damage. The most common illness is gastroenteritis, which causes stomach and intestinal inflammation and vomiting and diarrhea. The more serious viral water-borne illnesses can result in paralysis, neurological disorders, respiratory disease, heart problems, hepatitis, flu, warts, cancers, brain inflammation, kidney problems, and bladder diseases. My video presentation The Social Cost of Waterborne Viral Diseases From Bad Water Quality has more information.
Bacteria
Bacteria are likely the best-known classical organisms that can make you sick from drinking impure water. Bacteria are present in the intestines of all warm-blooded animals and are essential for digesting food. Lots of bacteria can make you sick. Common bacteria found in water include Escherichia coli (E. coli), Salmonella, Vibrio, Shigella, Campylobacter, Francisella, Legionella and Staphylococcus (8). These bacteria cause a wide range of diseases but gastroenteritis (GE) including stomach and intestinal inflammation, diarrhea, vomiting are probably the most common.
Protozoans
In Minnesota waters, the most common protozoan of concern is Giardia. This is the organism that can cause “beaver fever,” although beavers do not need to be involved. Giardia grow in the intestines of people and other animals and are transmitted to other animals via small cysts. An amoeba called Entamoeba histolytica causes very serious disease including the disease sometimes called amoebic dysentery. Its name is ominous. Histolytica means that an amoeba that’s in you bursts your cell, which is obviously unpleasant. Another protozoan of concern is Cryptosporidium that causes the diarrheal disease called cryptosporidiosis, or “crypto.” This one is hard to kill by water treatment. A rare but devastating protozoan is Naegleria fowleri, the so-called brain-eating amoeba which can be contracted via drinking water or swimming.
Worms
In general, worms are less common than viruses, bacteria, and protozoans, but the helminth worms can be very serious. These include Dracunculus and Fasciola. The former is the source of guinea-worm disease in the tropics and the latter is a flatworm or fluke that exists in both temperate and tropical waters. These organisms are about 1000 times longer than bacteria so can be somewhat easier to filter out or kill.
The cleaner the water, the easier to purify
Whatever water-purification method you use, a few limnology tips will help you get the best water possible. Remember that these harmful diseases and toxic materials come from people and land animals. Also remember that sediment from the bottom of a lake or waterbody can get suspended into the water by wind and wave action where the water is shallow.
You should take your water as far away from land and people as possible. Because sediment and colored water (like a tea stain) make water taste bad and interfere with purification, look for clear water that is unstained. Normally, this will be in the middle of a large lake. Large lakes have less tea staining because they have a higher pH than smaller, brown-colored lakes. Also, avoid taking your source water directly from the surface because materials you can’t see float at the surface. Limnologists (water scientists) get a purer sample by submerging their collecting containers to about arm’s length beneath the water surface.
If you are taking water from a river or stream take your water as far from the waters’ edge as possible and reach down to about halfway between the surface and the bottom. Water in mid-stream is refreshed most frequently and so will be of better quality than near the bottom, sides or surface.
Note that some people see water trickling out of rocks and think this a source of clean, deep groundwater. It might be but unless you know that this is a true-flowing spring (so-called artesian) and not just water trickling from land through cracks, you should assume that it is in close contact with land and animals and should perhaps be avoided. Avoid tea-stained water or cloudy water carrying sediments. Tea-stained water can be very harmful when purified using chlorination methods and the sediments can make you ill all by themselves. Also avoid water that has a bright green color to it as that may contain harmful algae.
Methods for purifying water in the wild
A few years ago, a group of wilderness doctors and scientists, members of the Wilderness Medical Society, wrote a very helpful review of the several methods used for water purification (9). They divide the purification or disinfection methods into four types: heat, ultraviolet light, filtration, and chemical treatment.
Heat (pasteurization)
Heating of liquids to eliminate living microbes has been done for hundreds of years. Louis Pasteur (1822-1895), a French scientist, first applied this method to preserving wine and beer to keep it from spoiling without destroying the character of those liquids (10). It is equally effective at purifying water and is the preferred method recommended by wilderness medical specialists (9). Pasteurization is a little different from the “boil water for 10 minutes” warnings that some specialists consider overkill (9). Bringing water to a boil (212 F) for one minute is usually plenty because organisms die during the heating process.
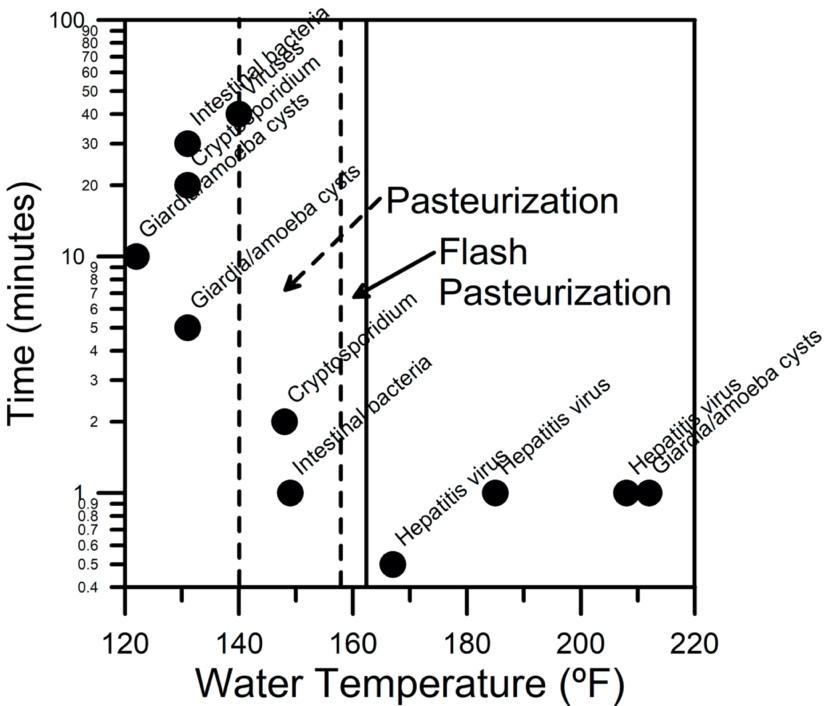
Boiling water when you are on a trail takes a lot of heating energy and the liquid gets so hot that it takes a long time to cool to a drinkable temperature. Instead, pasteurization heats the liquid to a warm temperature below the boiling point (140 F - 158 F) and holds it there for long enough to kill things that make you sick. Heating water to 158 F to 162 F will kill most dangerous organisms within about 30 seconds (11). A graph showing temperature-time combinations at complete death of various waterborne pathogens is in Figure 1. The graph shows that researchers indicate that bringing water up to a temperature of 160 F or higher for around a few minutes should be sufficient to rid the water of all dangerous organisms. Bringing water up to a boil should add even greater safety.
Pros and Cons
Heating is a time-tested and sure approach to preparing safe drinking water. It takes only a cooking pot, a source of heat, and a thermometer. Most people go camping with the first two items anyway and a serviceable, robust thermometer can be inexpensive. A perfect solution for the thermometer is a hot-drink thermometer, used for frothing coffee drinks (Figure 2). These are made of stainless steel, are tough, and cost between $3 and $12. A disadvantage is that it takes some planning ahead. For a three-person trip, that means heating 3-9 quarts (about 1-2 gallons) daily. Another disadvantage is that some people think that heated water tastes flat. This is because dissolved gases are driven out of the water during heating – shaking air back into the cooled water fixes that.
Ultraviolet light
If ultraviolet light strikes living organisms like bacteria, viruses and protozoans, it can kill them. This method is used for purifying water at the community and household level. It is energy intensive so has limited use in a wilderness setting. However, if the water is clear enough to allow light to penetrate, ultraviolet light can be used if other methods are unavailable. This method, called SODIS for solar disinfection, is being used around the world to decrease the incidence of diarrhea in developing countries (12). The method relies on clear plastic containers placed exposed to the sun for substantial periods of time.
What doesn’t work?
The most popular disposable bottles are often made of polyethylene terephthalate (PET), which is a material that is opaque to ultraviolet radiation.
What does work?
Clear plastic bottles made from materials like polypropylene, polycarbonate, polystyrene, polymethylmethacrylate and polyethylene bags, are preferred (12). Bags are more convenient to carry than bottles and H20How suggests carrying one-gallon, zipper closure freezer bags for SODIS.
Process
Bottles or bags should be partially filled, vigorously shaken to increase the oxygen concentration in the water, topped up, and left exposed to full sunlight for six or more hours with intermittent shaking (13) before consuming. In this method, ultraviolet action and solar heating combine to kill pathogenic organisms.
Pros and Cons
This method is very inexpensive to use. A disadvantage is that exposure time and full sunlight are needed for efficient operation and disinfection is best if the clear containers are left on a dark background. This means careful planning ahead. When one is traveling, this means that a substantial amount of water will need to be carried and, if the weather is overcast, it may be several days before disinfection will be reliable.
Filtration
Water filters are big business and complicated. The idea behind them is to strain the harmful organisms out of drinking water so they do not make you sick. Some filters also use methods that can take out harmful chemicals, as discussed in my Getting the Lead Out director's column on filtration of tap water in houses. Portable water filters use some pressure source (e.g., gravity, a pump, or suction from the mouth) to push water through a filter that has holes small enough that illness-causing organisms cannot pass through.
Filter sizes
There are three main sizes of filters used in commercially available water purification filters. The size measurement used to classify these is called a micrometer or micron (abbreviated µm). One micron is about 1/100th the thickness of a piece of paper.
Common filter sizes:
- Microfilters that filter out particles larger than about 0.1 µm
- Ultrafilters that filter particles larger than somewhere between 0.002-0.01 µm
- Nanofilters that filter out particles smaller than about 0.001 µm.
- Reverse osmosis filters remove particles that are even smaller than 0.001 µm (9).
Reverse osmosis filters require a power source or hand pump and may be slow. There are also some so-called sterilization filters with pore sizes of 0.2 µm. These are sometimes found in low-pressure field filtration units. Pleated paper whole household sediment filters generally remove particles greater than 1 µm. As pore size decreases, the pressure needed to push water through the filter increases greatly, so the cost and mechanics needed increase dramatically.
Catching gnats and elephants with the same net
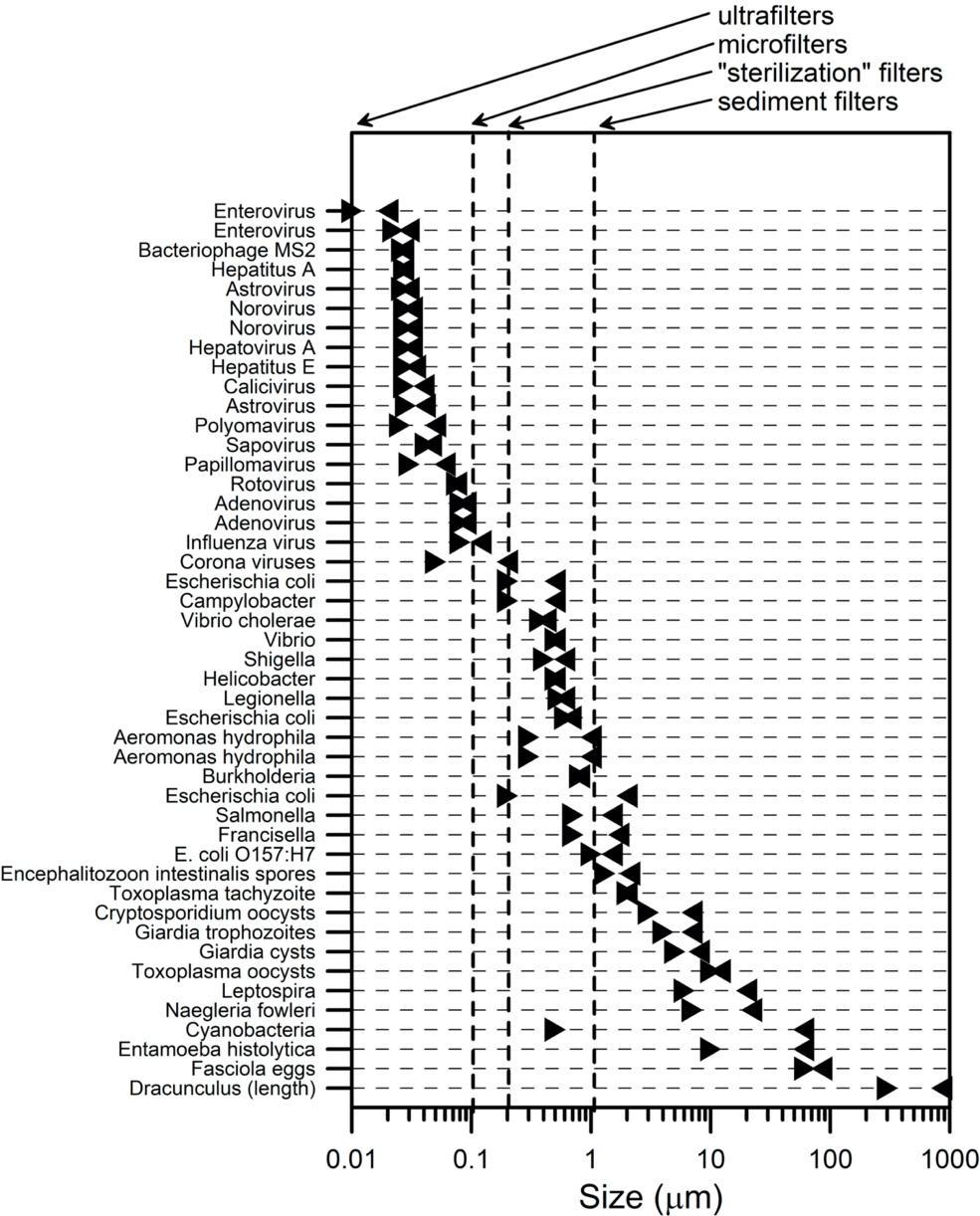
The size of particles a filter lets through makes a big difference because some waterborne pathogens are big, and some are small. Generally, it is a good idea to filter out as many of these things as possible. The sizes of waterborne pathogens of various sizes are shown in Figure 2. The vertical dashed lines show the sizes of openings in some common water filter types. Generally, organisms to the left of the dashed lines will not be removed by the type of filter indicated. Also, one should not assume that all the organisms to the right of vertical lines are removed because waterborne pathogens are malleable (flexible) and can squeeze through holes and slits smaller than their smallest dimension (14). For example, many waterborne bacteria can pass through sterilization filters with size ratings of 0.2 µm (15).
Figure 2 shows that although these illness-causing organisms are all small, the biggest of them can be 100,000 larger than the smallest of them. This is more than the difference in size between a gnat and an elephant. It is also greater than the difference in size between the blue whale, the largest animal on Earth, and the tiny krill they eat. No wonder engineers and designers have a difficult job. They are essentially trying to design nets that can be used to simultaneously capture all the elephants while not letting any gnats through the net.
The gnat-elephant problem is one reason filtration devices can fail to provide safety. If large particles clog the filter, water will no longer pass through it easily. This means that more pressure will be needed to get drinking water. High pressure and any kind of resulting damage can lead to breakage of the filter meaning that everything, including the accumulated filtered material can be delivered to your drinking water. Also, although some filters have materials such as silver in them meant to avoid pathogens, pathogens can still build up and grow inside the filter causing increased hazards. This is why one should treat filtration devices with care to avoid breakage and follow the manufacturer’s procedures for cleaning them.
A word about filter specifications. Look very carefully at the manufacturers’ claims. They are usually listed in the user’s manual or on the packaging. In a perfect world, these should be specific and backed up by real test results. They will nearly all claim some filter pore size, sometimes listed as µm, sometimes as microns and sometimes as micrometers. Compare this with Figure 2 to see what sort of organisms the filter pore size it is likely to remove. There is almost always some indication of the percentage removal of various types of organisms. What is important to note is that few claims made for filter size and efficacy are verified by any government testing agency, so you are trusting your health to the manufacturer.
Under U.S. EPA guidelines, “…a (filtration) unit, in order to be called a microbiological water purifier, must remove, kill or inactivate all types of disease-causing microorganisms from the water, including bacteria, viruses and protozoan cysts so as to render the processed water safe for drinking” (31). Under U.S. EPA guidelines, filters should reduce organisms in water by 99.9% for protozoa, 99.99% for viruses, and by 99.999%-99.9999% for bacteria (9). This means they can let through 1 out of every 1000 protozoans, 1 out of every 10,000 viruses, and 1 out of every 100,000-1,000,000 bacteria. But again, in the U.S. obtaining the evidence for this is the responsibility of the manufacturer.
The EPA does not test, approve or certify water filtration devices. The specifications for a product may also make some indication of the testing standard, i.e., NSF/ANSI 42 or NSF/ANSI 53. I discuss these standards at some length in my Getting the Lead Out director's column about household filters. In short, standard 42 only considers appearance and flavor issues about water whereas NSF/ANSI 53 addresses the removal of substances like microbes that can cause diseases. Some filtration units list other standards like NSF p231 or NSF p248. NSF p231 refers to a testing procedure outlined by the US-EPA in 1987 (31) and NSF p248 refers to a different testing protocol to simulate water purifier operation in military conditions. Most reputable manufacturers will show you a document specifying the tests their labs showed products to pass. However, unless an independent testing organization did the tests, you are still having to rely on the manufacturers’ claims. Products may also list a manufacturer’s assessment of the volume of water that can be treated without filter change The accuracy of those claims will vary greatly with the purity of the water source.
Pros and Cons
The main advantage of using water filters is that they can work quickly, offering drinking water that has at least some of the dangerous organisms removed and the water can be used right away. The main disadvantages are that they may not remove all the organisms that can make you sick, are expensive and sometimes bulky, can be fragile and fail, require maintenance to keep them working properly, and the disposable filters need to be replaced when they have lost filtration capacity, so generate trash. Further, your confidence in the outcome relies on your trust of the manufacturer.
Killing microbes with chemicals
Using chemicals to make water safer to drink cannot be called purification, because we are adding something to the water, so it is called disinfection. This is a bit like disinfecting a wound with iodine or disinfecting surfaces or clothing with chlorine bleach. In fact, using iodine and chlorine are two main ways to disinfect water by killing unhealthy organisms. Iodine and chlorine belong to a family of elements that chemists call halogens because they make (gen) salts (hal) when mixed with metals. Halogens have been known for decades to be deadly to microbes (and other living things) because they aggressively and permanently inactivate living cells (32). Much of domestic water treatment worldwide has been performed by adding chlorine to waters to irreversibly inactivate many types of harmful microbes. Since water disinfection is an active area of scientific research, there are also a few other chemical treatments available.
Time and disinfection
In all chemical treatments of drinking water, time, temperature and concentration matter. Scientists have done many tests to see how fast various organisms die when exposed to diverse concentrations of halogens and other chemicals. The idea is to use the lowest possible concentration while achieving disinfection in a convenient amount of time. Therefore, it is important to leave water exposed to the proper chemical concentration for long enough to achieve complete disinfection. Also, organisms are killed slower at low temperatures so longer waiting times are needed. Manufacturers’ instructions can be used as a guide to exposure time, but longer exposure time will generally achieve a higher degree of water disinfection.
Iodine treatments
Iodine has been used for water disinfection for many decades. It often imparts a golden color to the water and has some flavor that some people find objectionable. The most common forms that appear on the market are simple 2% tincture of iodine (tincture means iodine dissolved in ethyl alcohol and water), 10% iodine solution, iodine tablets (e.g., tetraglycine hydroperiodide) and iodinated resin (either triiodide or pentiodide) (33).
A 10% iodine solution needs to be added at about 50% higher concentrations than do tinctures. Removal of Giardia and other protozoan cysts takes very long contact times. This is why you probably know people who have chemically purified their drinking water and still contracted Giardia. Povidone-iodine solution is also thought to be effective although the evidence is less clear than it is for other iodine solutions (9). The use of iodine in water at high concentrations or for prolonged periods should be avoided as it can reveal and exacerbate thyroid diseases (34).
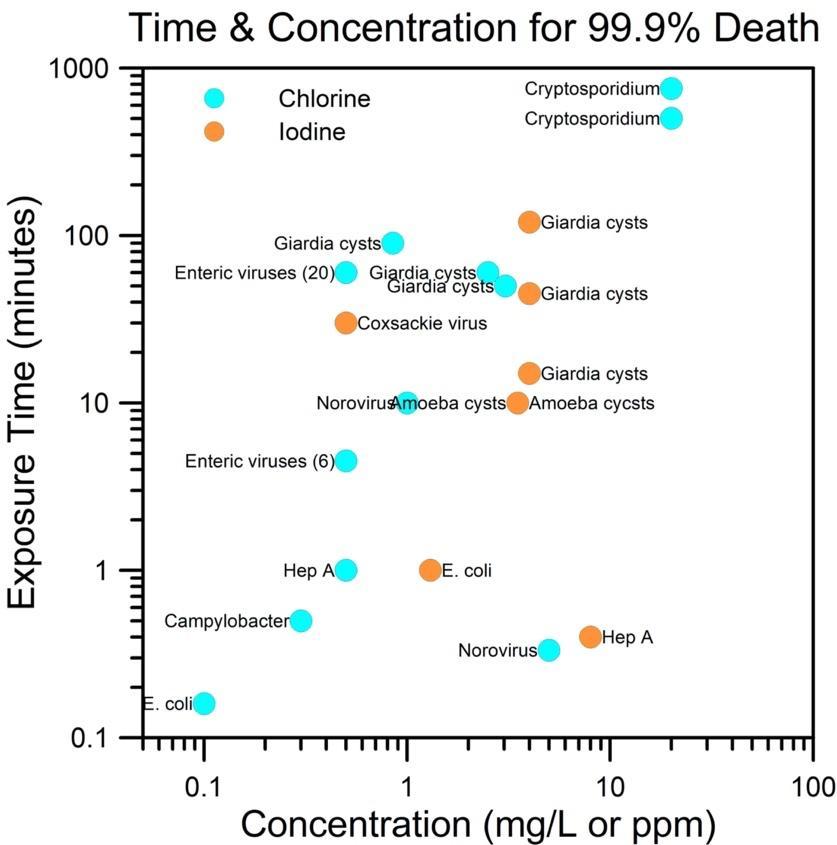
How many drops to add? Referring to Figure 4, about 5 drops of tincture of iodine per quart of water will yield 4 ppm of iodine and 10 drops will bring the concentration to 8 ppm. For 4 ppm you will need to add 8 drops of 10% povidone solution and 16 drops for 8 ppm (35).
Chlorine treatments
Chlorine treatment is probably the approach that keeps your city’s domestic water supply free of pathogens. For killing pathogens in the field, the most common chemical forms are 5% sodium hypochlorite (unscented household bleach), sodium chlorite, sodium dichloroisocyanurate and calcium hypochlorite. According to the World Health Organization (WHO) (33), about 4 drops of unscented household bleach per quart creates a solution of about 10 ppm (35) and can be effective if contact times are long enough (see Figure 4). This could take 12-14 hours to kill protozoans but would kill most bacteria and protozoans after 60-90 minutes.
Do not use chlorine-based chemicals if you are trying to disinfect brown or tea-stained water. This is because chlorine (and iodine to a lesser extent) can combine with the dissolved molecules forming the stain and create dangerous substances called disinfection by-products or DBPs. The DBPs have been associated with high rates of bladder cancer, miscarriage and cell abnormalities in people (36).
Removing the halogen taste
Some people avoid iodine or chlorine disinfection because of the taste imparted to the water. Backer et al. (9) suggest that this taste can be removed easily by adding a small pinch of ascorbic acid (vitamin C) to a quart of water after the appropriate contact time with the disinfectant. This converts the chlorine to chloride and the iodine to iodide so that neither will have an objectionable flavor. Ascorbic acid is available in most pharmacies and is a principal ingredient in powdered drink mixes.
Miscellaneous chemical treatments
A drinking water disinfectant that is gaining popularity is chlorine dioxide (9). This disinfectant is more effective than any other against Cryptosporidium (35). Because these disinfectants rely on some complex chemical reactions, they often are sold as two-part treatments. Hydrogen peroxide has been suggested as a water disinfectant but it is caustic and has been subjected to fewer reliable tests.
Pros and Cons
Chemical disinfection can be inexpensive and effective using any of the chemicals, if contact time is long enough. Because they need to disrupt cells, some of the larger organisms are harder to kill with chemicals, and take very long contact time. Because you are adding one or more chemical substances to your drinking water, flavor may be altered but can usually be corrected with small amounts of vitamin C or other methods.
Summary
Decisions about the most appropriate method of water purification are matters of personal choice and a person’s own assessment of relative risks and benefits. Because hydration is so important, you should never have just one method available when you are far from civilization. Water pasteurization is a simple and effective method to eliminate all pathogens quickly but requires a heating source, cooling time, and a thermometer. Ultraviolet purification takes quite long sanitation times and relies on substantial sunshine and being stationary for long enough to kill microbes. Chemical treatments are inexpensive but require long contact times to kill some of the larger and dangerous pathogens. They also impart a chemical flavor to water that some find objectionable but can be corrected inexpensively. Water filtration is the most costly and complex method and can require a lot of study to make sure the filter is suited to the microbes you want to protect yourself from. This is made more difficult because recreational waters are seldom tested for pathogens and one never knows what might be present. Whatever method you decide to use, give yourself a head start by choosing the clearest, most sediment- and color-free water, collected as far from sources of human, sediment and animal contact as possible.
References and Notes
- J. S. Yoder, G. F. Craun, R. L. Calderon, Surveillance for waterborne disease and outbreaks associated with recreational water use and other aquatic facility-associated health events--United States, 2005-2006. (Centers for Disease Control and Prevention (CDC), US Department of Health …, 2008), vol. 57.
- T. R. Burch et al., Statewide Quantitative Microbial Risk Assessment for Waterborne Viruses, Bacteria, and Protozoa in Public Water Supply Wells in Minnesota. Environmental Science & Technology 56, 6315-6324 (2022).
- M. S. Islam, M. Hassan-uz-Zaman, M. S. Islam, J. D. Clemens, N. Ahmed, in Waterborne Pathogens, M. N. Vara Prasad, A. Grobelak, Eds. (Butterworth-Heinemann, 2020), pp. 1-14.
- E. Jéquier, F. Constant, Water as an essential nutrient: the physiological basis of hydration. European Journal of Clinical Nutrition 64, 115-123 (2010).
- B. M. Popkin, K. E. D'Anci, I. H. Rosenberg, Water, hydration, and health. Nutrition Reviews 68, 439-458 (2010).
- J. N. Morris, D. G. Clayton, M. G. Everitt, A. M. Semmence, E. H. Burgess, Exercise in leisure time: coronary attack and death rates. British Heart Journal 63, 325 (1990).
- M. N. Sawka, S. N. Cheuvront, R. I. Carter, Human Water Needs. Nutrition Reviews 63, S30-S39 (2005).
- D. N. Magana-Arachchi, R. P. Wanigatunge, in Waterborne Pathogens, M. N. Vara Prasad, A. Grobelak, Eds. (Butterworth-Heinemann, 2020), pp. 15-42.
- H. D. Backer, R. W. Derlet, V. R. Hill, Wilderness Medical Society Clinical Practice Guidelines for Water Disinfection for Wilderness, International Travel, and Austere Situations. Wilderness & Environmental Medicine 30, S100-S120 (2019).
- J. H. Steele, History, trends, and extent of pasteurization. Journal of the American Veterinary Medical Association 217, 175-178 (2000).
- M. F. Islam, R. B. Johnston, Household pasteurization of drinking-water: the chulli water-treatment system. J Health Popul Nutr 24, 356-362 (2006).
- Á. García-Gil, R. A. García-Muñoz, K. G. McGuigan, J. Marugán, Solar Water Disinfection to Produce Safe Drinking Water: A Review of Parameters, Enhancements, and Modelling Approaches to Make SODIS Faster and Safer. Molecules. 2021 (10.3390/molecules26113431).
- K. G. McGuigan et al., Solar water disinfection (SODIS): A review from bench-top to roof-top. Journal of Hazardous Materials 235-236, 29-46 (2012).
- K. D. Young, Bacterial Shape: Two-Dimensional Questions and Possibilities. Annual Review of Microbiology 64, 223-240 (2010).
- S. Sundaram, S. Mallick, J. Eisenhuth, G. Howard, H. Brandwein, Retention of Water-Borne Bacteria by Membrane Filters. Part II: Scanning Electron Microscopy (SEM) and Fatty Acid Methyl Ester (FAME) Characterization of Bacterial Species Recovered Downstream of 0.2/0.22 Micron Rated Filters. PDA Journal of Pharmaceutical Science and Technology 55, 87 (2001).
- R. D. Adam, The biology of Giardia spp. Microbiological Reviews 55, 706-732 (1991).
- Z. Altintas, M. Gittens, J. Pocock, I. E. Tothill, Biosensors for waterborne viruses: Detection and removal. Biochimie 115, 144-154 (2015).
- L. Atmar Robert, K. Estes Mary, Diagnosis of Noncultivatable Gastroenteritis Viruses, the Human Caliciviruses. Clinical Microbiology Reviews 14, 15-37 (2001).
- C. A. Cleveland et al., The wild world of Guinea Worms: A review of the genus Dracunculus in wildlife. International Journal for Parasitology: Parasites and Wildlife 7, 289-300 (2018).
- J. P. Dubey, D. S. Lindsay, C. A. Speer, Structures of Toxoplasma gondiiTachyzoites, Bradyzoites, and Sporozoites and Biology and Development of Tissue Cysts. Clinical Microbiology Reviews 11, 267-299 (1998).
- G. W. Harrington, I. Xagoraraki, P. Assavasilavasukul, J. H. Standridge, Effect of Filtration Conditions On Removal of Emerging waterborne pathogens. Journal AWWA 95, 95-104 (2003).
- T.-C. Li et al., Characterization of Self-Assembled Virus-Like Particles of Merkel Cell Polyomavirus. PLOS ONE 10, e0115646 (2015).
- H. Mach et al., Disassembly and reassembly of yeast‐derived recombinant human papillomavirus virus‐like particles (HPV VLPs). Journal of Pharmaceutical Sciences 95, 2195-2206 (2006).
- S. K. Maciver, J. E. Piñero, J. Lorenzo-Morales, Is Naegleria fowleri an Emerging Parasite? Trends in Parasitology 36, 19-28 (2020).
- C. R. Madeley, Comparison of the Features of Astroviruses and Caliciviruses Seen in Samples of Feces by Electron Microscopy. The Journal of Infectious Diseases 139, 519-523 (1979).
- P. S. Masters, in Advances in Virus Research. (Academic Press, 2006), vol. 66, pp. 193-292.
- P. Pushko et al., Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 23, 5751-5759 (2005).
- T. Solomon et al., Virology, epidemiology, pathogenesis, and control of enterovirus 71. The Lancet Infectious Diseases 10, 778-790 (2010).
- M. A. Valero, N. A. n. Darce, M. Panova, S. Mas-Coma, Relationships between host species and morphometric patterns in Fasciola hepatica adults and eggs from the northern Bolivian Altiplano hyperendemic region. Veterinary Parasitology 102, 85-100 (2001).
- Y. Wang, F. Hammes, M. Düggelin, T. Egli, Influence of Size, Shape, and Flexibility on Bacterial Passage through Micropore Membrane Filters. Environmental Science & Technology 42, 6749-6754 (2008).
- C. P. Gerba, S. A. Schaub, "Guide Standard and Protocol for Testing Microbiological Water Purifiers," (United States Environmental Protection Agency, Washington, DC, USA, 1987).
- D. E. Green, P. K. Stumpf, The Mode of Action of Chlorine. Journal (American Water Works Association) 38, 1301-1305 (1946).
- O. World Health, Guidelines for drinking-water quality: first addendum to the fourth edition. (2017).
- H. Backer, J. Hollowell, Use of iodine for water disinfection: iodine toxicity and maximum recommended dose. Environmental Health Perspectives 108, 679-684 (2000).
- C. D. Ericsson, R. Steffen, H. Backer, Water Disinfection for International and Wilderness Travelers. Clinical Infectious Diseases 34, 355-364 (2002).
- C. J. Williams, D. Conrad, D. N. Kothawala, H. M. Baulch, Selective removal of dissolved organic matter affects the production and speciation of disinfection byproducts. Science of The Total Environment 652, 75-84 (2019).